The most widely used surface treatment process for aluminum and aluminum alloys is anodic oxidation. After anodic oxidation, an oxide film of a few microns to several hundred microns can be produced on the surface, which has significantly improved corrosion resistance, wear resistance, decorative properties, insulation, and heat dissipation compared to the natural oxide film of aluminum alloys.
What is Anodic Oxidation?
Aluminum alloy products are anodized in an electrolyte solution.The process of forming an aluminum oxide film on the surface of aluminum alloy by electrolysis is called anodic oxidation of aluminum alloy. If not specified, anodizing is generally referred to as sulfuric acid anodizing.
The process of anodic oxidation.
Degreasing → alkaline corrosion (also called alkali bite) → polishing/drawing/sandblasting → chemical polishing → black film stripping → anodizing → dyeing pretreatment → dyeing → sealing → drying
- Degreasing (degreasing) can remove the cutting fluid, oil, grease, etc. attached to the surface of the workpiece during the cold working process;
- Alkali bite is to remove the excess grease and remove the natural oxide film, burrs, and impurities on the surface through the saponification of NaOH;
- Before anodizing the surface, brushing or sandblasting can make the surface lusterless while polishing can make the surface shiny. In addition, chemical polishing can remove surface impurities, natural oxide film, dirt, etc., so that the product is shinier.
- In phosphoric acid polishing, the surface will remain a layer of gray film, therefore, the need to use nitric acid stripping black film.
The appearance of anodized colors.
Any color can be achieved by anodizing except white. The anodizing method is called the “universal” technology for the surface treatment of aluminum alloys.
The anodic oxidation of aluminum and aluminum alloy is the process of forming an aluminum oxide film on the surface of aluminum or aluminum alloy products by placing them in an electrolyte solution as an anode. The thickness of the anodic oxide film of aluminum and its alloys can reach several tens to hundreds of microns, which not only has good mechanical properties and corrosion resistance, wear resistance, and weather resistance, but also has strong adsorption properties.
Aluminum products can be electrolytically colored after anodic oxidation. With the growth of color time, the color can be produced from light to dark, and a variety of colors can also be produced, with a beautiful decorative appearance that can be obtained after using various coloring methods.
Properties of anodic oxidation.
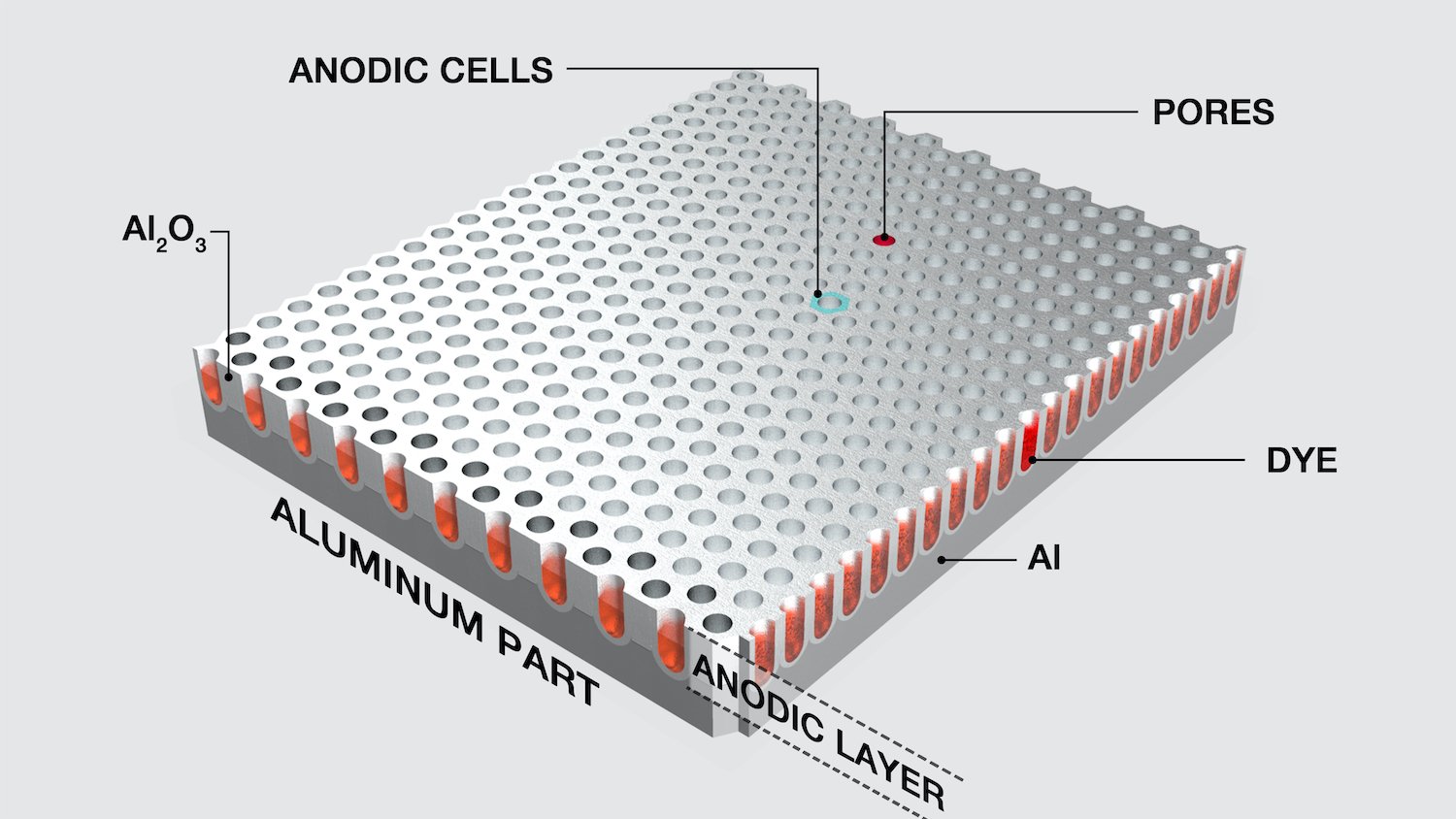
- Porosity – The oxide film has a porous honeycomb structure, and the void rate of the film layer is determined by the type of electrolyte and the process conditions of oxidation. The porous structure of the oxide film can make the film layer show good adsorption ability to various organic substances- resin, ground wax, inorganic substances- dyes, paints, etc. It can be used as the base layer of the coating layer, and the oxide film can also be dyed into various different colors to improve the decorative effect of the metal.
- Wear resistance – Aluminum oxide film has high hardness, which can improve the wear resistance of the metal surfaces. When the film layer adsorbs lubricant, it can further improve its wear resistance.
- Corrosion resistance – Aluminum oxide film is stable in the atmosphere, so it has good corrosion resistance. Its corrosion resistance is related to the thickness of the film, composition, void ratio, composition of the substrate material, and integrity of the structure. In order to improve the corrosion resistance of the film, the anodized film is usually closed or painted again.
- Electrical insulation – Anodized film has high insulation resistance and breakdown voltage and can be used as a dielectric layer of electrolytic capacitors or an insulation layer of electrical products.
- Adiabatic – Aluminum oxide film is a good adiabatic layer, and its stability can reach 1500℃, so the parts working under an instant high temperature can prevent the melting of aluminum due to the presence of the oxide film.
Quick Links:
🔗Detailed Tour of FEIYU Factory – Environment
https://www.youtube.com/watch?v=xRmfUuPDwxM&ab_channel=FeiyuSmart_Official